 Other Ingredients: Hypromellose, Magnesium Stearate, FD & C Red #40, Titanium Dioxide, FD & C Blue #1 Other Ingredients: Hypromellose, Magnesium Stearate, FD & C Red #40, Titanium Dioxide, FD & C Blue #1| NDC 52747-0711-30 | | 30 | | Capsules | | Rx only | | | NDC 52747-0711-60 | | 90 | | Capsules | | Rx only | |
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. | |
You should contact your healthcare provider for medical advice about adverse events. To report a serious adverse event, contact US Pharmaceutical Corporation, P.O. Box 360465, Decatur, GA 30036. INDICATIONS: Integra F™ is indicated for the treatment of iron deficiency anemia and folate deficiency as in extended convalescence, menorrhagia, pregnancy, puberty, excessive blood loss and advanced age. Also for treatment of condition in which iron deficiency and vitamin C deficiency occur together, as well as cases of metabolic stress, and in convalescence.
CONTRAINDICATIONS: Integra F™ is contraindicated in patients with a known hypersensitivity to any of its ingredients; also, all iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis, or hemolytic anemias. It is also contraindicated in patients suffering from pernicious anemia as folic acid may obscure its signs and symptoms.
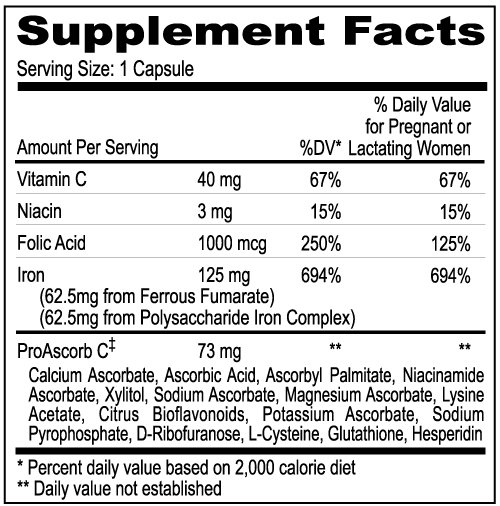
Ferrous Fumarate and Polysaccharide Iron Complex: All Integra™ products include a unique patented source of iron, e.g. Ferrous Fumarate and Polysaccharide Iron Complex (U.S. Patent No: 11/243,043 Pending). "An increase in tolerability is observed with the (patented formulation) and is believed to occur as the result of distributing the total iron content in the composition among compounds that provide iron to the patient's blood stream via two different mechanisms. The ferrous salts are readily absorbed in the upper gut, by direct dissolution and absorption of the ferrous iron by the bloodstream. However, the iron available from PIC is absorbed in the lower gut, via an active protein transport mechanism".
Clinical Studies: Picinni, L.-Ricciotti, M. 1982. Therapeutic effectiveness of an iron-polysaccharide complex in comparison with iron fumarate in the treatment of iron deficiency anemias: PANMINERVA MEDICA-EUROPA MEDICA, Vol. 24, No. 3, pp. 213-220 (July-September 1982).
Folic Acid: Folic Acid is one of the important hematopoietic agents necessary for proper regeneration of the blood-forming elements and their function. Additionally, folic acid increases jejunal glycolytic enzymes and is involved in the desaturation and hydroxylation of long-chain fatty acids in the brain. A deficiency in folic acid results in megaloblastic anemia.
WARNING: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient.
PRECAUTIONS: Folic acid in doses above 0.1 mg - 0.4 mg daily may obscure pernicious anemia, in that hematological remission can occur while neurological manifestations remain progressive.
OVERDOSE: Iron: Signs and Symptoms: Iron is toxic. Acute overdosage of iron may cause nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other symptoms include pallor and cyanosis, melena, shock, drowsiness and coma. The estimated overdose of orally ingested iron is 300-mg/kg body weight. When overdoses are ingested by children, severe reactions, including fatalities, have resulted.
DOSAGE AND ADMINISTRATION: Adults (persons over 12 years of age), one (1) capsule daily, between meals, or as prescribed by a physician. Do not exceed recommended dosage. Do not administer to children under the age of 12.
HOW SUPPLIED: Integra F™ are maroon capsules imprinted "US" logo and "Integra-F" in white. Child resistant bottles of 90 capsules, NDC# 52747-711-60, and 30 capsules, NDC# 52747-711-30. Dispense in a tight, light-resistant container as defined in the USP/NF with a child resistant closure. Store at controlled room temperature 15° to 30°C (59° to 86° F). Keep in a cool, dry place.
CAUTION: Rx only. Rev. 06/2013
|