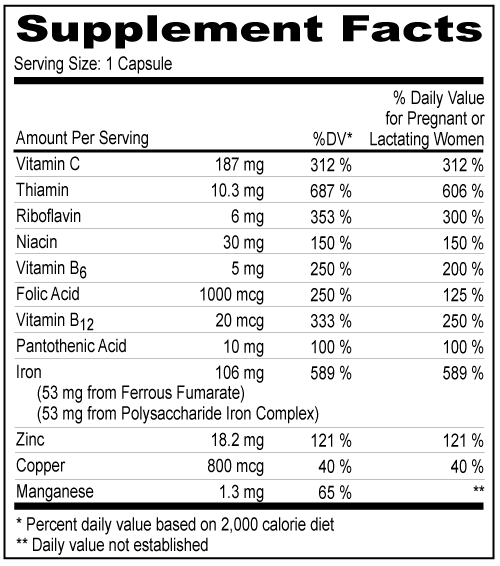
 Other Ingredients: Sodium Ascorbate, Gelatin, Zinc Sulfate, Niacinamide, D-calcium Pantothenate, Thiamin Mononitrate, Silicon Dioxide, Pyridoxine HCl, Magnesium Stearate, Manganese Sulfate, Titanium Dioxide, Copper Sulfate, Dibasic Calcium Phosphate, FDA/E172 Red Iron Oxide, Cyanocobalamin Other Ingredients: Sodium Ascorbate, Gelatin, Zinc Sulfate, Niacinamide, D-calcium Pantothenate, Thiamin Mononitrate, Silicon Dioxide, Pyridoxine HCl, Magnesium Stearate, Manganese Sulfate, Titanium Dioxide, Copper Sulfate, Dibasic Calcium Phosphate, FDA/E172 Red Iron Oxide, Cyanocobalamin| NDC 52747-0902-60 | | 90 | | Capsules | | Rx only | |
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. | |
WARNING: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. You should contact your healthcare provider for medical advice about adverse events. To report a serious adverse event, contact US Pharmaceutical Corporation, P.O. Box 360465, Decatur, GA 30036. Marketed by US Pharmaceutical Corporation, P.O. Box 360465, Decatur, GA 30036. INDICATIONS: Tandem® Plus is indicated for the treatment of iron deficiency anemia and folate deficiency as normally found in extended convalescence, menorrhagia, pregnancy, puberty, excessive blood loss and advanced age. Also for correction of condition in which iron deficiency and vitamin C deficiency occur together, along with a deficient intake or increased need for B-Complex vitamins in chronic and acute illness, as well as cases of metabolic stress, and in convalescence.CONTRAINDICATIONS: Tandem® Plus is contraindicated in patients with a known hypersensitivity to any of its ingredients; also, all iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis, or hemolytic anemias. Pernicious anemia is a contraindication, as folic acid may obscure its signs and symptoms.PRECAUTIONS: General: Folic acid in doses above 0.1 mg - 0.4 mg daily may obscure pernicious anemia, in that hematological remission can occur while neurological manifestations remain progressive.
Pediatric Use: Safety and effectiveness of this product have not been established in pediatric patients.
Geriatric Use: No clinical studies have been performed in patients age 65 and over to determine whether older persons respond differently from younger persons. Dosage should always begin at the low end of the dosage scale and should consider that elderly persons may have decreased hepatic, renal, or cardiac function and or concomitant diseases. Adverse Reactions: Folic Acid: Allergic sensitizations have been reported following both oral and parenteral administration of folic acid.
Ferrous Fumarate: Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally, but are usually mild and may subside with continuation of therapy. Although the absorption of iron is best when taken between meals, giving Tandem® Plus after meals may control occasional G.I. disturbances. Tandem® Plus is best absorbed when taken at bedtime.
OVERDOSE: Iron: Signs and Symptoms: Iron is toxic. Acute overdosage of iron may cause nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other symptoms include pallor and cyanosis, melena, shock, drowsiness and coma. The estimated overdose of orally ingested iron is 300-mg/kg body weight. When overdoses are ingested by children, severe reactions, including fatalities, have resulted. Tandem® Plus should be stored beyond the reach of children to prevent against accidental iron poisoning. Keep this and all other drugs out of the reach of children.
Treatment: For specific therapy, exchange transfusion and chelating agents should be used. For general management, perform gastric lavage with sodium bicarbonate solution or milk. Administer intravenous fluids and electrolytes and use oxygen.
DOSAGE AND ADMINISTRATION: Adults (persons over 12 years of age), One (1) capsule daily, orally, between meals, or as prescribed by a physician. Do not exceed recommended dosage. Do not administer to children under the age of 12.
HOW SUPPLIED: Tandem® Plus are pink capsules imprinted radially "Tandem-Plus" and "US, US, US, US/US, US, US, US": Child resistant bottles of 90 capsules NDC# 52747-902-60. Dispense in a tight, light-resistant container as defined in the USP/NF with a child resistant closure. Store at controlled room temperature 15° to 30°C (59° to 86° F). Keep in a cool, dry place. Capsules are not USP.
CAUTION: Rx only. Rev. 04/2011
|